Ingredients
The basic structure of the isoflavones is characterized by its 2-phenylchromane ring (flavan) which consists of two aromatic rings that are linked with different substituents such as methoxyl, hydroxyl or sugar via a tetrahydopyran ring.
The isoflavone content varies in the individual parts of the red clover plant with different concentrations being found in the flower, the leaves and the stem. The total concentration of isoflavones is described as being between 4.627% and 6.566% mg/g dw (dry weight) in the literature (Krenn L et al., 2002; Saviranta NM et al., 2008; Tsao R et al., 2006; Wu Q et al., 2003).
MF11RCE® contains a defined ratio of the 4 most important isoflavone subtypes: formononetin, genistein, biochanin A and daidzein. Formononetin is a methylated predecessor of daidzein, while biochanin A is a methylated predecessor of genistein.
Structural formula
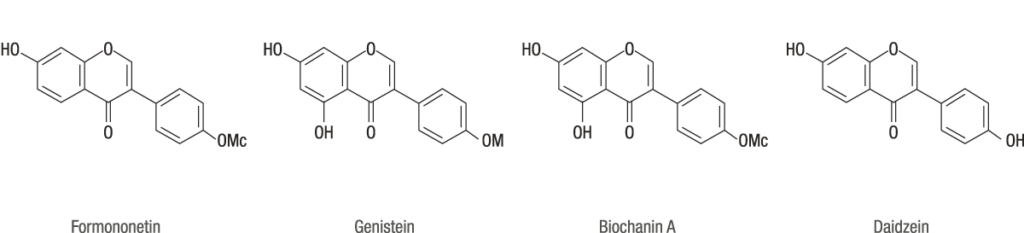
(Genistein+biochanin A)
——————————— = 0,9 to 1,7
(Daidzein+formononetin)
In order to obtain this standardized extract, the plants are cultivated in Europe and are controlled according to strict standards that conform with Good Agricultural Practices (GAP). MF11RCE® is harvested at a time and in such a way that there is an optimal concentration of isoflavones.
The isoflavones contained in MF11RCE® are present as biologically active aglycones, meaning that no additional cleavage is required in the intestine and absorption into the bloodstream is easier. This is of crucial importance for the pharmacokinetics of MF11RCE®.
Administration and dosage
Administration: orally.
Dosage: 80 mg of MF11RCE® in a once-daily dose.
Side effects
Side effects tend to be the exception when MF11RCE® is taken properly, but cannot be ruled out completely. Attention should be paid in case of very rare, yet possible intolerances or allergic reactions to isoflavones.
The side effects of taking red clover isoflavones reported in the literature, such as headaches, myalgia and vomiting, are classified as mild and rare (Geller SE et al., 2006).
In the MF11RCE® studies carried out up until now, no adverse effects were observed.
Safety of use
In a prospective, double-blind, randomized, placebo-controlled crossover study, Lipovac M et al. (2006) investigated the effect of MF11RCE® on selected sex hormones and the endometrium. Following supplementation with 80 mg of MF11RCE®, the estradiol serum values remained unchanged and the endometrial thickness was reduced. The study confirmed the efficacy of an 80 mg daily dose of MF11RCE® for postmenopausal women. Complementing earlier isoflavone studies, it supplied further evidence for the safety of using MF11RCE® in relation to estrogen-like effects, such as an increased cancer risk for hormone-dependent glandular tissue (endometrial hyperplasia) and other contraindications for hormone replacement therapy.
In 2015, the long-term safety of isoflavones was confirmed by the EFSA. A comprehensive risk assessment by the European Food Safety Authority (EFSA), which evaluated numerous human studies, concluded that there is no sign of unwanted effects on hormone-dependent tissue such as breast, uterus or thyroid. The long-term safety was confirmed for a daily dose of up to 150 mg of isoflavones over a period of at least 3 years (EFSA 2015).
