M. Imhof , A. Gocan, F. Reithmayr, M. Lipovac, C. Schimitzek , P. Chedraui, J. Huber
Effects of a red clover extract (MF11RCE®) on endometrium and sex hormones in postmenopausal women
Published in Maturitas 2006 55: 76-81
Number of patients: 109
| Study Setting | MF11RCE® Group | Control Group |
| Inclusion Criteria | Postmenopausal women (amenorrhea >12 months), 40 years or older with moderate-to-severe menopausal symptoms (Kupperman index ≥15) | Postmenopausal women (amenorrhea >12 months), 40 years or older with moderate-to-severe menopausal symptoms (Kupperman index ≥15) |
| Exclusion Criteria | positive pregnancy test, nonwillingness,on hormonal therapy (HT), known isoflavone hypersensitivity | positive pregnancy test, nonwillingness,on hormonal therapy (HT), known isoflavone hypersensitivity |
| Parameter | Serum levels of testosterone (T), 17-estradiol (E2), follicle stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone binding globulin (SHBG); endometrial thickness. | Serum levels of testosterone (T), 17-estradiol (E2), follicle stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone binding globulin (SHBG); endometrial thickness. |
| Treatment | MF11RCE® 80 mg for a 90-day period. After a 7 day washout period, subjects switched to receive the opposite treatment for another 90 days. | Plazebo for a 90-day period. After a 7 day washout period, subjects switched to receive the opposite treatment for another 90 days. |
Introduction
MF11RCE® is considered a highly interesting alternative to ERT, which is particularly true for a subgroup of phytoestrogens, isoflavones. Isoflavones exhibit estrogenic activity by ER-activation and anti-estrogenic activity by competitive binding to ER and inhibiting E2. Moreover,they have a significantly higher affinity to ER-β than to ER-α. Due to the heterogenous distribution of the two ER subforms, isoflavone activity is stronger in tissues rich in ER-β, e.g. bone, capillary epithelium, skin, and central nervous system.
Aim of the Study
The aim oft the study was to evaluate the effects of MF11RCE® on selected sex hormones and endometrium in postmenopausal women.
Materials and Methods
Verum was 80 mg MF11RCE® red clover extract, with a standardized content of aglyconic isoflavones in form of biochanin A, formononetin, genistein and daidzein. Examinations comprised anamnesis, medication anamnesis, body weight, blood pressure and transvaginal ultrasound. Fasting blood samples were taken for analysis. These examinations were performed before and after both treatment phases. Outcome measures: Serum levels of testosterone (T), 17-estradiol (E2), folliclestimulating hormone (FSH), luteinizing hormone (LH), and sex hormone binding globulin (SHBG); endometrial thickness.
Patients & Study Design
This prospective randomized, double-blind, placebocontrolled trial was carried out by the Study Center Med XIX, Vienna, Austria, and the Department for Gynecological Endocrinology and Reproductive Medicine, General Hospital, Vienna, Austria.
One-hundred and thirteen women were recruited from the daily routine.
Inclusion criteria were: postmenopausal status (amenorrhea > 12 months), 40 years or older, negative pregnancy test , moderate to severe menopausal symptoms (Kupperman index ≥15).
Women under constant ERT or with a known isoflavone hypersensitivity were not accepted.
Participants were randomly assigned to one of two groups to receive two capsules of either MF11RCE® (verum) or placebo for a 90 day period. After a 7 day washout period, subjects switched to receive the opposite treatment for another 90 days.
109 women were accepted for evaluation, 50 assigned to group A and 59 to group B. The two groups were comparable.
Statistical Analysis
Statistical analysis was performed on an intention-to-treat basis using software SPSS11.0 (SPSS Inc., Chicago, IL).
Due to different absolute values at the baseline points of the two phases, comparison was performed with regard to the observed changes. Differences between verum and placebo phases were assessed by Wilcoxon rank test. Changes within each of the treatment phases were assessed using paired Ttest. A p-value ≤ 0.05 was considered statistically significant.
Results
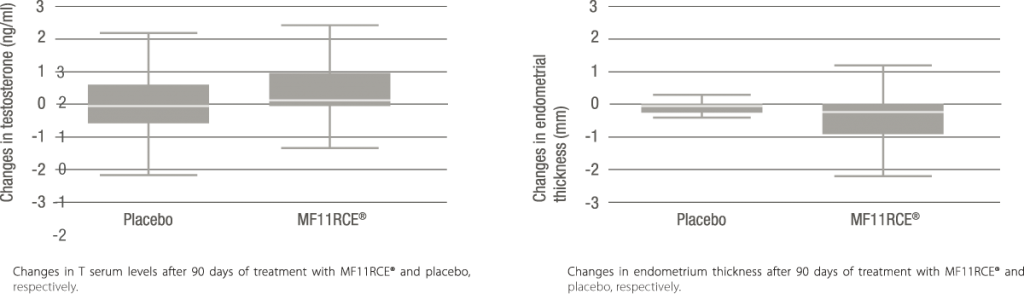
The evaluation of changes with verum and placebo versus baseline revealed a significant increase in T serum levels of about 22% (p < 0.001) and a significant decrease in endometrial thickness of 14.7%
(p < 0.001) with verum, but not with placebo. No effects were found for E2, FSH, and SHBG
Discussion
The present double-blind, randomized, cross-over study demonstrated a significant effect of 80mg MF11RCE® over T-levels and endometrial thickness, while exerting no effect over E2, FSH, and SHBG levels when compared to baseline or placebo.
The reduction of endometrial thickness is an interesting finding in the context of safety considerations, which is a major concern in the treatment of menopausal disorders, particularly in terms of suspected adverse effects of ERT. Isoflavones have been suggested as a promising alternative because they seem
to avoid undesired estrogen-related effects.
Conclusion
MF11RCE® exerts a moderate effect on testosterone levels, a significant effect on endometrium thickness in postmenopausal women, while estradiol levels remained unchanged.
Practical Benefits
In postmenopausal women, MF11RCE® supplementation may
- moderately improve serum testosterone levels and, thus, mood state,
- have no effect on serum estradiol levels,
- moderately reduce endometrial thickness and
- thus, provide further evidence of safety of MF11RCE®-isoflavones in terms of hormone like effects, a.o. endometrial hyperplasia.